On this week’s episode of CW’s The Flash, “Escape from Earth-2,” the Flash (Earth-1’s Barry Allen) was Zoom’s prisoner, held inside a cell with transparent walls that he couldn’t escape. By the way, the episode is still available to watch at The CW’s website.
On this week’s episode of CW’s The Flash, “Escape from Earth-2,” the Flash (Earth-1’s Barry Allen) was Zoom’s prisoner, held inside a cell with transparent walls that he couldn’t escape. By the way, the episode is still available to watch at The CW’s website.
Zoom could, as we saw, move back and forth through the walls, but when Barry tried vibrating through them, he bounced off. Barry’s reasoning was that he wasn’t vibrating fast enough, and later, that he wasn’t vibrating at the right frequency.
Look, we’re not really spoiling anything to say that Barry eventually escapes, but before that – that’s the part we’re interested in. Just prior to Barry getting out, the rest of Team Flash showed up, including Earth-2’s Harrison Wells. As Killer Frost tried to freeze (and shatter) the wall of Barry’s cell…nothing happened. Looking to Dr. Wells for help, the the scientist explained, “Carbyne. It’s some form of carbine. The cell’s made out of carbyne. You’ll never be able to freeze him out of there.”
 Whaaaaat? Carbyne? Did The Flash writers just make up a new material that is a stand-in for “stuff that Flash can’t get through, but Zoom can?”
Whaaaaat? Carbyne? Did The Flash writers just make up a new material that is a stand-in for “stuff that Flash can’t get through, but Zoom can?”
Actually, no.
Sure, when Wells says it, and the Flash is standing behind a wall of it, “carbyne” takes on a science-fiction air, but in reality, carbyne is real stuff. Score one on the science side for The Flash writers – who actually do put in some nice real-world science now and again…mixed in with healthy doses of the made up, but we’re not here to judge. We’re here to talk about what carbyne is.
As the name suggests, carbyne has something to do with the element carbon.
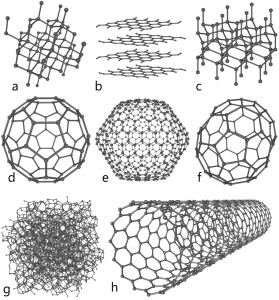
A quick carbon review: thanks to the configuration of its electrons, atoms of carbon are able to form four bonds with other atoms. When carbon bonds with itself, it forms different structures called allotropes. Carbon’s allotropes are made of 100% carbon atoms, but the atoms are arranged in different patterns relative to one another. This isn’t some lab-coat fascination science – you know the three most common: amorphous (powdered) carbon – just clumps of atoms hanging around together, graphite – look at your pencil, carbon atoms arranges in sheets which come off when rubbed against something like paper, and diamonds. Other allotropes include versions you may have heard of – buckyballs, graphene, fullerenes, carbon nanotubes…all made up solely of carbon, but different in the way the atoms bond and the number of bonds between them.
Off all the allotropes, graphene is the big sexy lately. It’s a honeycomb lattice of carbon atoms one atom thick. At it’s simplest, it’s a sheet of atoms. It has length and width – two dimensions. Two neat things about graphene – 1) you can make your own and; 2) If you roll a sheet of graphene into a cylinder that’s still one atom thick, you’ve got a carbon nanotube.
Long story short, carbon is the wonder atom and can exist in many different forms when it gets together with itself. Which brings us to carbyne.
Carbyne is a chain of single carbon atoms that are bonded to each other with double bonds, or alternating single and triple bonds (each bond is a shared pair of electrons between the two atoms). Since it’s only one atom “thick,” and an atom is the smallest piece of matter that’s still that particular matter – in this case, carbon, carbyne is seen as being one-dimensional. In other words, it only has length and no width.
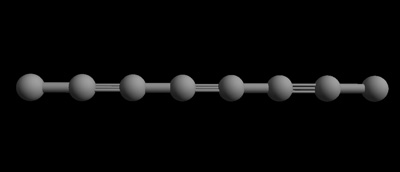
Carbyne with 8 carbon atoms — a one dimensional chain of carbon atoms with alternating single and triple bonds. (Edinformatics.com)
As a result of it’s particular structure, carbyne has a tensile strength – a resistance to being stretched – that’s higher than any other known material, even diamonds, graphene and carbon nanotubules. It’s safe to call it the strongest material known.
While carbyne research is just getting started (the most often cited paper was published in 2013), the idea of carbyne has been around for decades, and the material itself has been found in compressed graphite, in meteorites and interstellar dust.
The properties of carbyne are weirdly interesting too – normally, the chain of carbon atoms are stiff and relatively inflexible, but that can change depending on what’s attached at the ends. Add, for instance, methylene molecules to the ends of one, and the carbyne chain can twist. Additionally, the chains don’t like to get too close to each other thanks to an energy barrier that keeps them apart – although bundles of chains may stick to each other.
And as an aside to Killer Frost – current research hasn’t investigated how carbyne behaves at very low temperatures, but it probably would be able to lose a lot of heat (that is, get cold) before it would show any effects.
So – making this stuff, just in case you need to set up a cell for holding a speedster from a parallel earth? It’s…tricky. To date, synthesizing carbyne has been slow and difficult, with the largest chains (as of about three years ago) only reaching 44 atoms long. Most construction guys will tell you that’s not quite enough to make a wall.
Carbyne is truly a nanoscale material at this point – produced and one day used in a scale of billionths of meters.
Maybe we should call the Atom…